Parkinson’s Disease
Parkinson’s disease (PD) is a progressive neurodegenerative disorder affecting nearly one million Americans and approximately 10 million people worldwide.

Current treatments provide symptomatic relief, but none modify the underlying progression of the disease. Most individuals with PD initially respond to dopamine replacement therapy, yet for the majority of patients, over time, this type of treatment is no longer sufficiently helpful while adverse effects of medication can also occur, leading to a considerable reduction in quality of life and the ability to function effectively.
Our approach:
We are developing AAV-GAD for treatment of Parkinson’s disease.
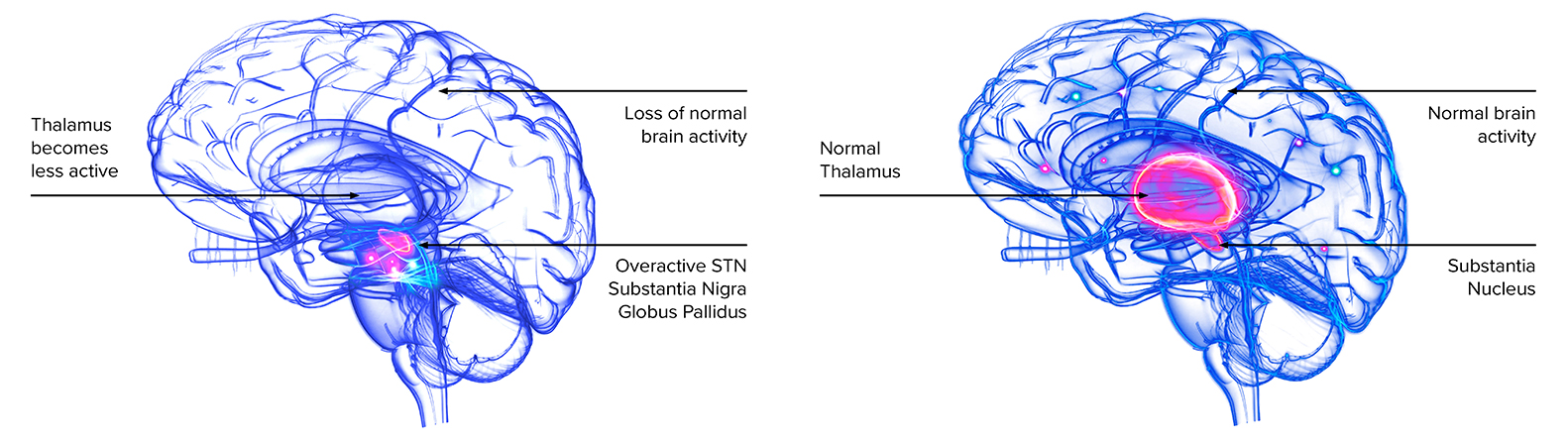
AAV-GAD is designed to reprogram dysfunctional brain circuits through the local production of GABA in the subthalamic nucleus. GABA is a chemical neurotransmitter that can help restore more normal activity of the subthalamic nucleus, a key regulator of the circuits responsible for normal movement.
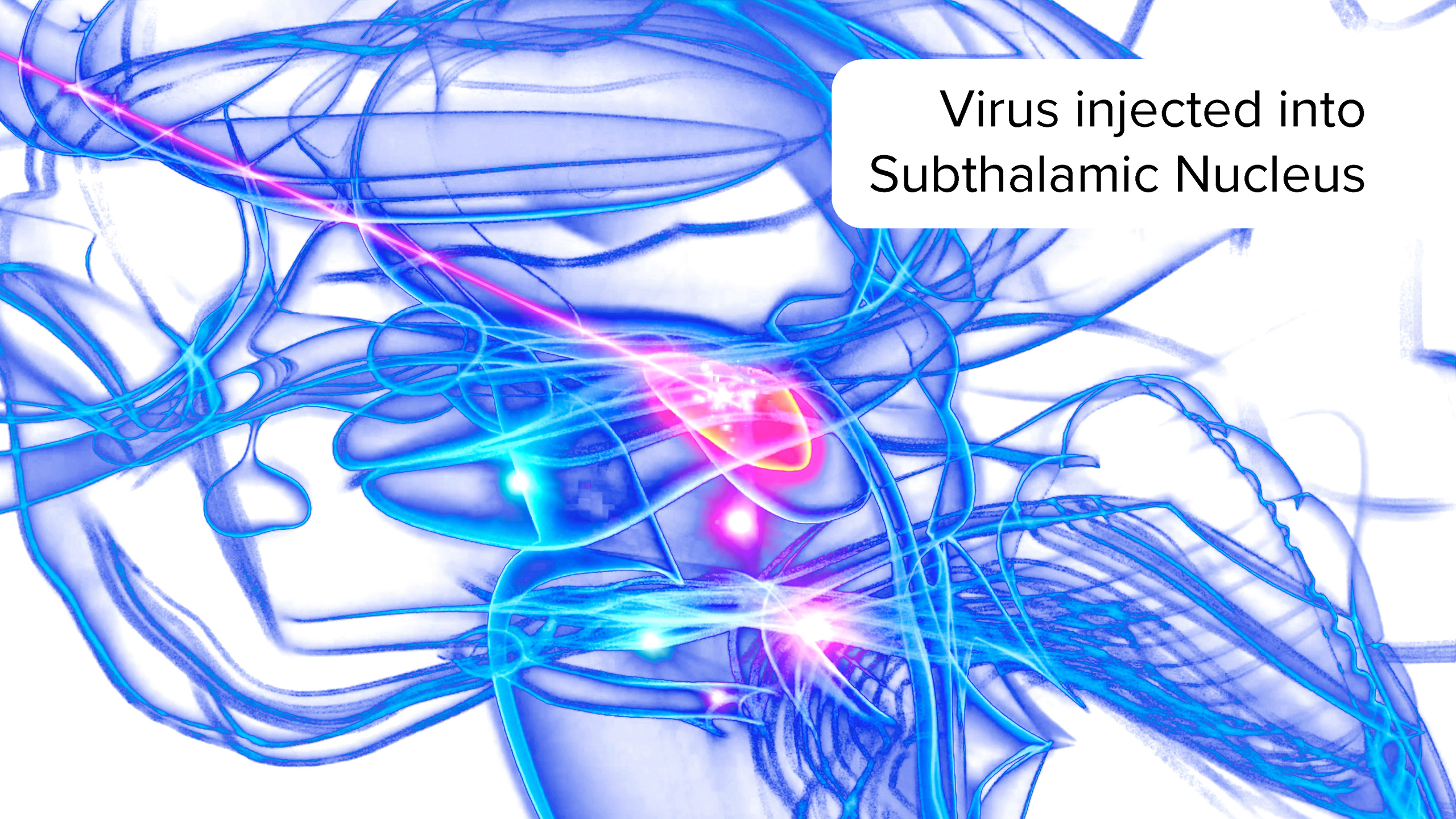
AAV-GAD is delivered via a one-time infusion through a minimally invasive procedure, using a MeiraGTx proprietary device that allows infusion of the equivalent of one drop of gene therapy solution into the subthalamic nucleus.
AAV-GAD was studied in three human clinical studies and is the only CNS gene therapy program with two randomized, double-blind, sham surgery controlled trials which met a prespecified primary endpoint.
AAV-GAD has been tested in 58 patients across three clinical studies and is the only cell or gene therapy in PD to meet the prespecified primary endpoint in two randomized, double-blind, sham surgery-controlled trials.
To learn more about the promising results from MeiraGTx’s Parkinson’s AAV-GAD trials to date, please see our corporate presentation here.
MeiraGTx was Granted FDA Regenerative Medicine Advanced Therapy (RMAT) Designation for AAV-GAD for the Treatment of Parkinson’s disease (see the full announcement here)
- This RMAT designation is based on data from 3 clinical studies demonstrating the potential benefit of AAV-GAD as a one-time treatment for Parkinson’s disease
- RMAT designation includes the benefits of the Fast Track and Breakthrough Therapy designations, allows frequent regulatory interactions with the FDA, and potential routes to accelerated approval and Priority Review
