Radiation-Induced Xerostomia (RIX)
Xerostomia (dry mouth) is a condition in which a person’s salivary glands do not produce enough saliva. Symptoms include difficulty eating, chewing, and speaking, oral pain, sore throat, difficulty sleeping, inability to exercise, uncontrollable dental caries (tooth decay), yeast infections, oral burning and inability to wear dentures.

Xerostomia has a number of different potential causes but may result from radiation therapy for head and neck cancer or be caused by certain autoimmune diseases such as Sjogren’s disease. Currently, there are no effective treatments for people with moderate or severe xerostomia.
Our Approach:
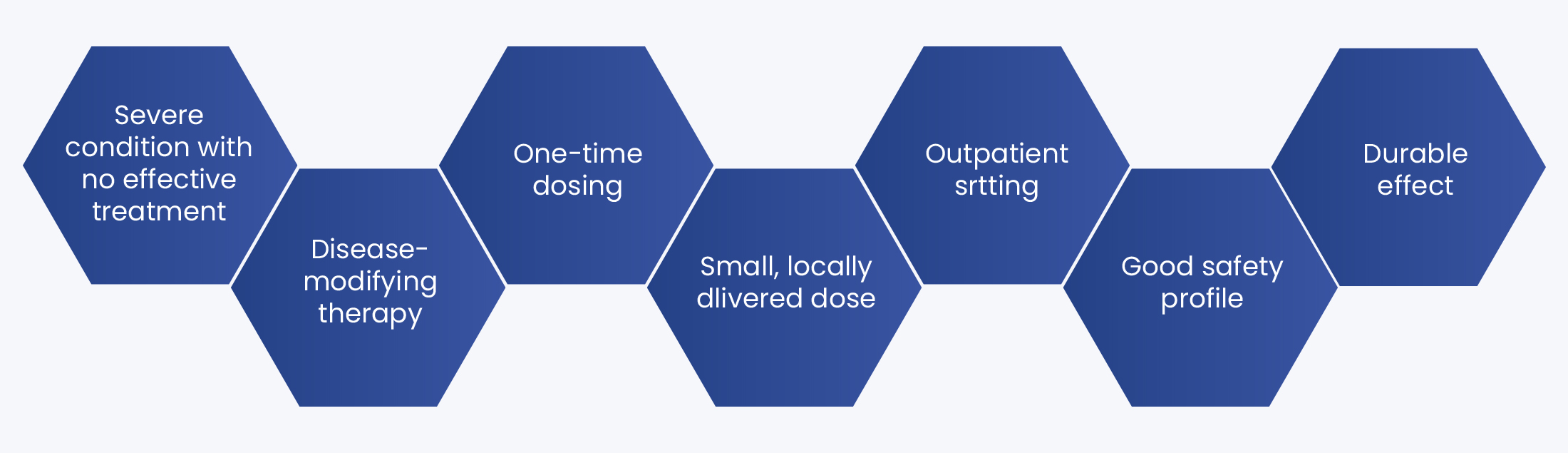
We are developing AAV-AQP1 to treat radiation-induced xerostomia (RIX). AAV-AQP1 is an investigational genetic medicine that is designed to increase water conduction in salivary glands that have been damaged by radiation therapy.
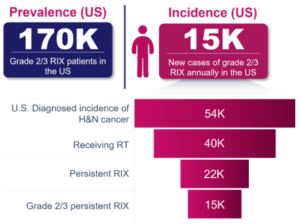
Worldwide, approximately 650,000 new cases of head and neck cancer are diagnosed each year, with approximately 54,000 cases in the U.S. Eighty-five percent of radiation-treated patients reported reduced saliva production, of whom 40% have persistent Grade 2/3 RIX. In the U.S., RIX is believed to affect approximately 170,000 patients.
AAV-AQP1 introduces a water conducting channel into the damaged glands. We are currently conducting a Phase 2, potentially pivotal study for people with moderate or severe dry mouth (xerostomia) caused by radiation therapy for head and neck cancer, the AQUAx2 Study.
AAV-AQP1 may be appropriate for treatment of xerostomia resulting from other conditions, such as Sjogren’s Syndrome or PSMA radioligand therapy.
To learn more about the promising results from MeiraGTx’s Xerostomia AAV-AQP1 trials to date, please see our corporate presentation and our June 2023 news release here.
MeiraGTx was Granted FDA Regenerative Medicine Advanced Therapy (RMAT) Designation for AAV2-hAQP1 for the Treatment of Grade 2/3 Radiation-Induced Xerostomia (see the full announcement here)
- RMAT designation recognizes the preliminary clinical evidence of the potential benefit of AAV2-hAQP1 as a one-time treatment for this debilitating condition
- RMAT designation includes the benefits of the Fast Track and Breakthrough Therapy designations, allows frequent regulatory interactions with the FDA, and potential routes to accelerated approval and Priority Review
