Manufacturing
Over the past decade, cell and gene therapies have revolutionized how serious diseases can be treated and potentially cured. Prior to gene-based therapies many of these diseases were effectively untreatable. The realization of these promising therapies has come through scientific advances in a range of disciplines including molecular biology, gene regulation, protein engineering and production methodologies. Manufacturing science and technologies are critical components in the development of gene therapies and their safe and effective clinical application to patients who need them most.
Every step in the manufacturing process is essential to having a safe and effective GMP product:
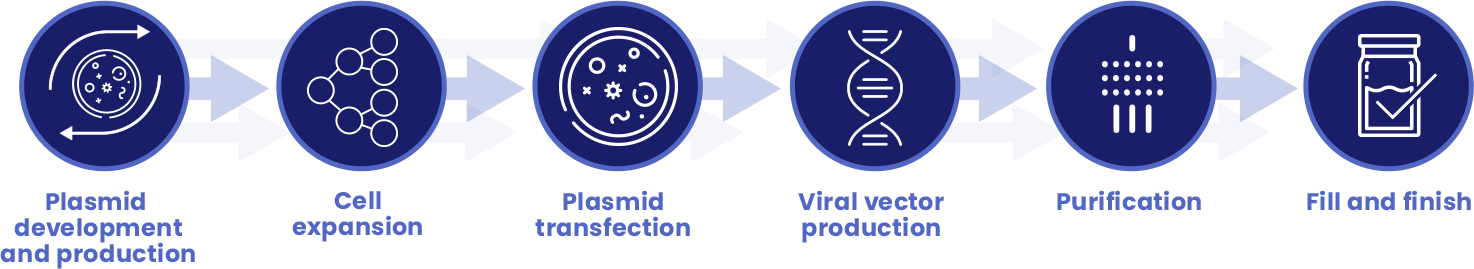
With more than 180,000 square-feet of combined dedicated manufacturing facilities in London, UK and Shannon, Ireland, MeiraGTx has brought all aspects of manufacturing and process development in-house. With manufacturing as a fully integrated vertical component of the Company, our capabilities have allowed us to alleviate one of the most significant bottlenecks in the clinical development process as well as reduce regulatory risk and ensure the delivery of safe, potent, and effective products, all while decreasing our cost of goods.
Comprised of cell expansion, bulk production virus and downstream purification suites, fill and finish and quality control laboratories, our cGMP certified viral vector manufacturing facility in London is designed to support a platform manufacturing process which can be readily adapted for parallel production of multiple viral vector systems and products at multiple scales.
Additionally, our recently completed plasmid and DNA production facility in Shannon has allowed us to internalize plasmid and DNA manufacturing, a key impediment in the supply chain and a significant cost component in manufacturing supply.
Combined, MeiraGTx’s facilities offer the flexibility and scalability to support the supply of innovative gene therapy product candidates at all stages of the product development cycle, from pre-clinical stages through clinical trials and commercialization.
Our manufacturing sciences, production and quality team consists of more than 180 highly trained multidisciplinary staff with backgrounds in a diverse array of manufacturing sciences, technologies, analytics and production. Our specialized team is working to expedite the delivery of gene therapy products and is committed to ensuring MeiraGTx’s platform process continues to be at the forefront of this industry and is focused on quality and production efficiency to ensure we deliver the safest and most effective science to our patients.



“Gene therapy is a next-generation medicine that requires novel manufacturing technologies in order to be both flexible and scalable. Flexibility allows us to take our process and apply it to new medicines and address markets that we previously would not have been able. The efficiency and simplified scale-up allow for shorter production time and increases our ability to potentially help a greater number of patients.”
– Stuart Naylor, Ph.D., Chief Development Officer

